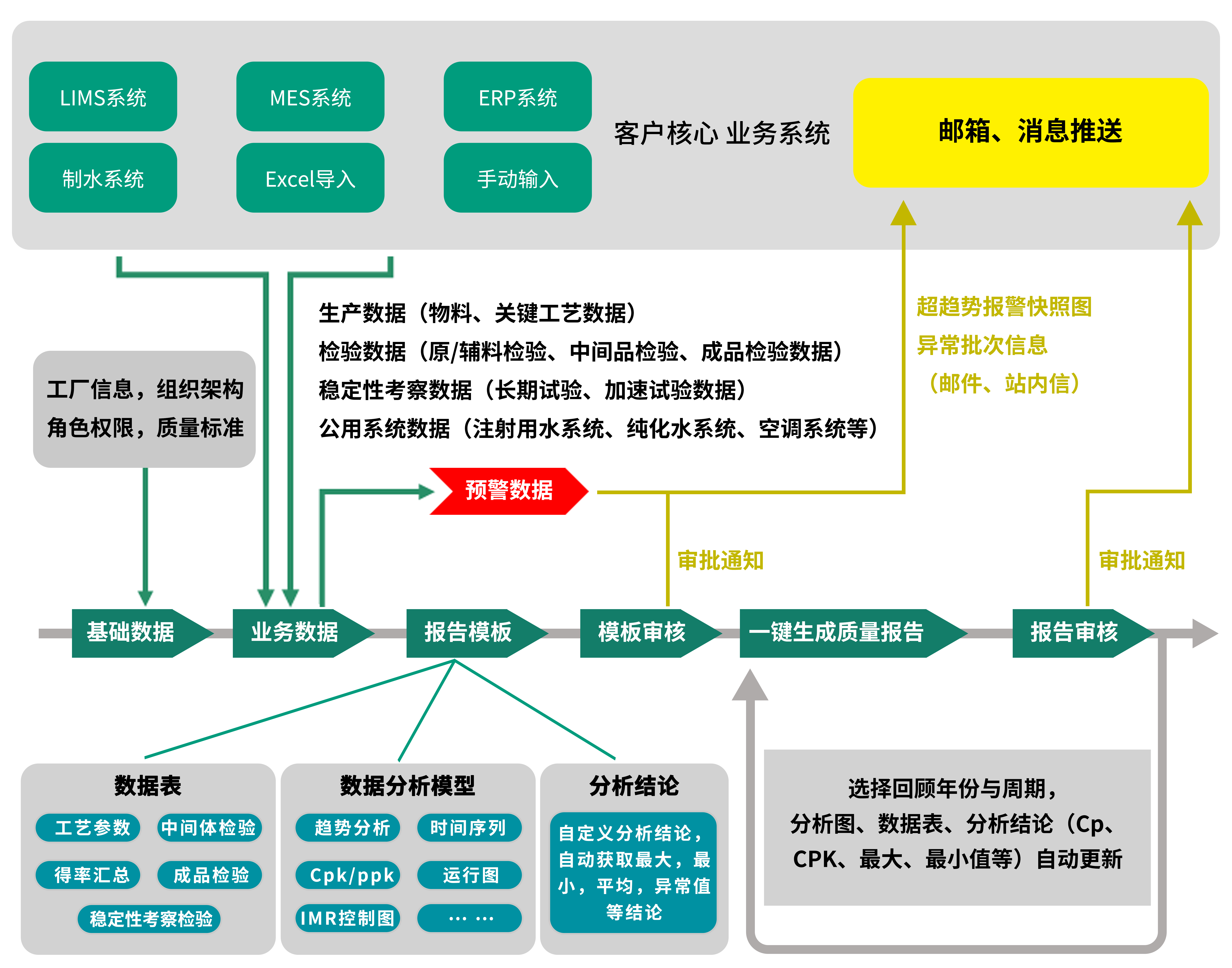
Utilize the existing informatization system (interface
docking) or regularly maintained Excel spreadsheets (Excel import) to aggregate
production/inspection/stability investigation/public system data into the
Tigermed QRCS. Combined with the data analysis model built into the system,
monthly/quarterly/annual quality review reports can be generated with one
click. This fundamentally solves problems such as scattered data, difficult
collection, and lack of analysis capabilities in the process of making review
reports. It effectively alleviates the production pressure on the enterprise's
quality department in making review reports and significantly reduces the
production cycle. By using various analysis models in the system, value-added
functions such as business data early warning, horizontal/vertical comparative
analysis, and two-way traceability can also be realized.
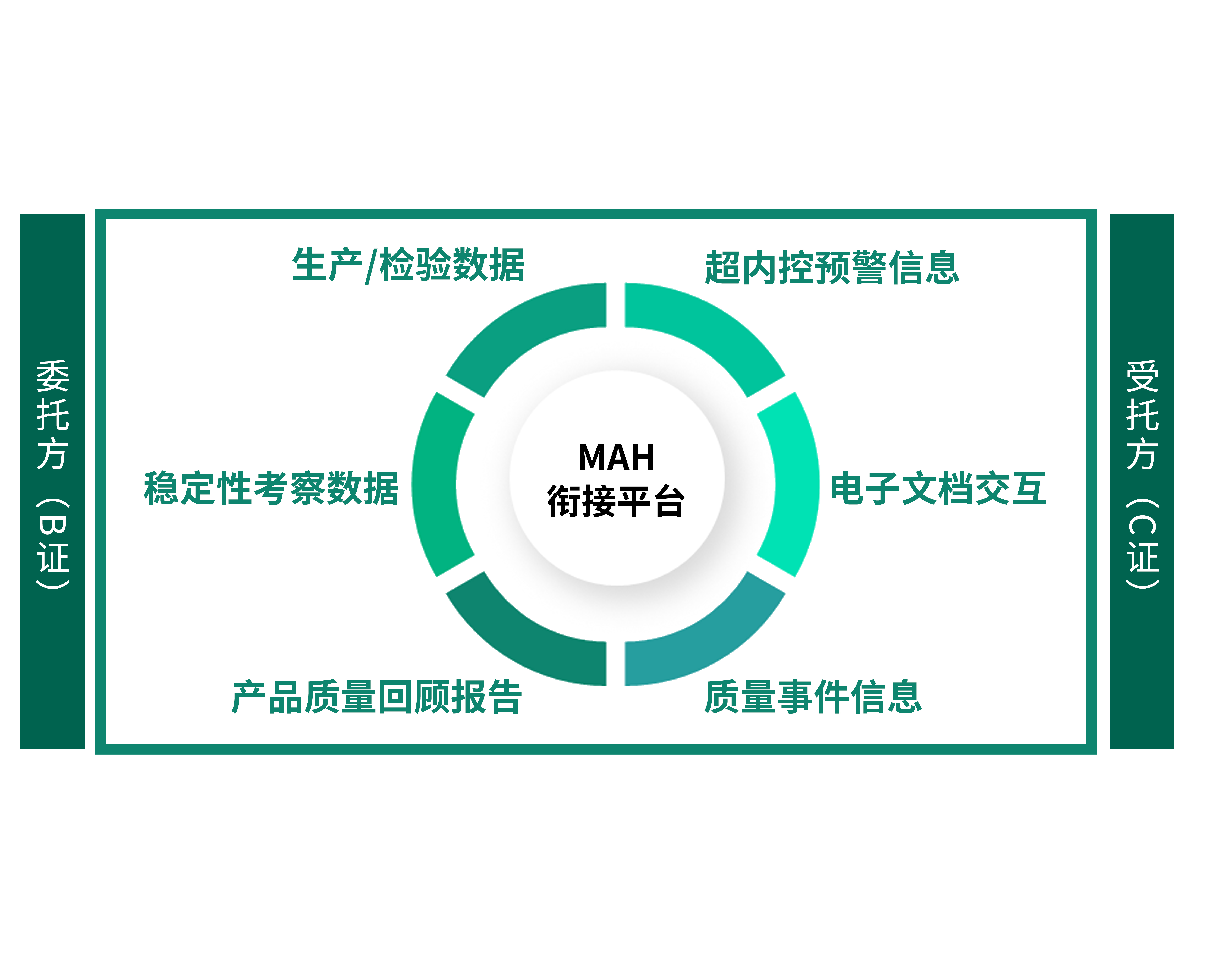
Through the MAH connection platform of the Tigermed Quality
Risk Control System, the review, transfer, and data archiving of production and
inspection process documents and records between different
commissioners/consignees can be realized. For example: production data,
inspection data, stability investigation data, early warning information
exceeding internal control, product quality review reports
(annual/quarterly/monthly), quality event information (deviation, change, CAPA,
OOS/OOT), etc., and online approval of interactive documents (production plans,
technical documents), etc. Become your professional B/C license connection
platform.
致力于赋能药企质量&研发&生产信息化、数字化转型









